Before choosing a testing method, consider the organism of interest and the purpose of testing.
I. Qualitative testing
Reports whether a target organism is present or absent. Often used for detecting pathogens. Due to their rarity, pathogen tests rely on enrichment techniques, e.g. special growth media to enhance target organism growth while suppressing non-target microbes. Because multiplication rates are unknown, results are binary, i.e. presence/absence.
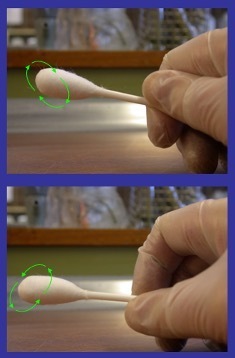
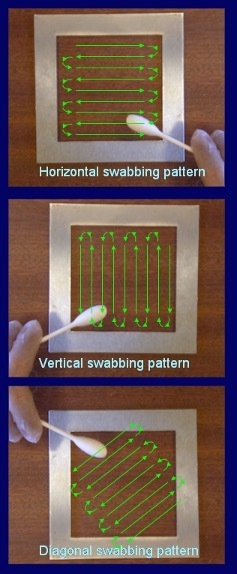
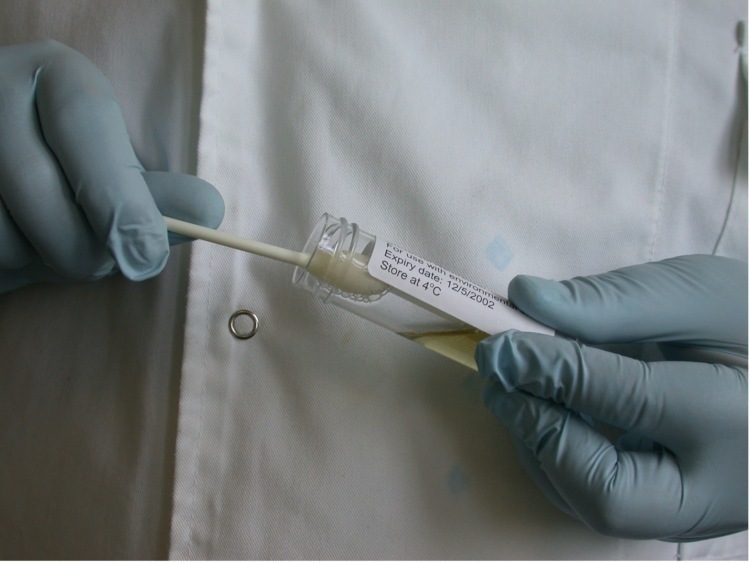
Pathogen testing typically requires larger sample areas, often using sponges rather than small swabs (1).
II. Quantitative testing
Provides numerical counts of bacterial cells and is commonly used to verify cleaning and disinfection effectiveness. It typically involves indicator organisms, such as aerobic mesophiles, faecal coliforms, or Enterobacteriaceae. Indicator tests help assess the hygienic status of surfaces. The Total Count of Aerobic Mesophiles (TCAM), also known as Total Viable Count (TVC), is widely used and cost-effective.
Table 1: Common indicator bacteria and their significance
| Indicator bacteria | Additional details |
|---|
| Thermotolerant Escherichia coli | Growth at ≥42ºC indicates recently deposited faecal material. Growth at ≤25ºC generally indicated the presence of older faecal material. |
| Thermotolerant faecal coliforms | A subset of the Enterobaceriaceae that are associated with the gastrointestinal tracts of humans and animals. Growth at ≥42ºC indicates recently deposited faecal material. Growth at ≤25ºC generally indicated the presence of older faecal material. |
| Enterobacteriaceae | The Enterobacteriaceae are a group of bacteria that include many species, including E. coli. The group grow at a range of temperatures including enterics (around 40ºC), mesophiles (around 25C) and phychrotrophs (≤4ºC). The Enterobacteriaceae tend to be found growing in rotting vegetation, faeces, water, soil, and gastrointestinal tracts. Most of them do not cause illness. Where they come from, the environments in which they grow well and the way they die is the same as for most food foodborne pathogens they are used as a general hygiene indicator for more harmful bacteria Because they come from a range of environmental sources and grow over a range of temperatures, the Enterobacteriaceae are a general hygiene indicator of more harmful bacteria. The Enterobacteriaceae are widely used to check the microbiological effectiveness of thermal death of bacteria in cooked food. |
| Enterococci | General indicators of faecal contamination |