What's on this page:
This guidance is for food businesses in Scotland producing products of animal origin (POAO). It outlines the health and identification mark requirements for POAO produced by Scottish businesses placed on Great Britain, Northern Ireland, EU and non-EU markets.
Identification marks
The identification mark is applied to POAO to show it has been produced in an establishment approved in accordance with food safety and hygiene regulations and is typically applied to wrapping, packaging, or labelling which contains, or is attached to, the POAO.
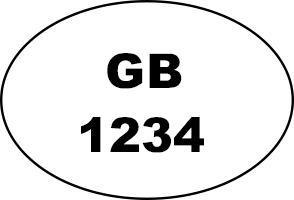
Table 1 shows a summary of which identification marks may be used for POAO produced and placed on the market in Great Britain and Northern Ireland, or exported outside of the UK.
| Health marks | Establishment where mark is applied | Great Britain market | Northern Ireland market | EU 27 market | Non-EU market |
|---|---|---|---|---|---|
 |
Great Britain - FSS approved establishments |
Yes | Yes | Yes | Yes* |
 |
Great Britain - FSS approved establishments |
Yes | Yes | Yes | Yes* |
 |
Great Britain - FSS approved establishments |
Yes | No | No | Yes* |
* These apply from 01 January 2021
Size and dimension of identification marks
The identification mark must:
- be legible and indelible, with the characters easy to read
- contain either the ‘GB’ or ‘UK’ abbreviation or the full country name ‘United Kingdom’ for POAO produced in Great Britain
There is no minimum or maximum size for the identification mark.
Adjustment period for changes to identification marks for POAO placed on the Great Britain market
The 36-month adjustment period will expire on 31 December 2023. During this period it has been possible for approved food businesses to use existing stocks of labels, wrapping and packaging bearing the ‘UK/EC’ identification mark (ID Mark) for products being placed on the GB market. After 31 December 2023, food businesses in Great Britain cannot continue to apply labels containing the ‘UK/EC’ identification mark to products of animal origin.
For Northern Ireland, specific conditions for health and ID marking have been set out in the Northern Ireland Protocol which forms part of the UK-EU Withdrawal Agreement.
Read FSA guidance on health and ID marking in Northern Ireland.
Health marks
The health mark is an official mark which is applied to carcases to show they were processed in an establishment approved in accordance with food safety and hygiene regulations and that the carcass itself is fit for human consumption.
Table 2 shows a summary of which health marks may be used for POAO produced and placed on the market in Great Britain and Northern Ireland, or exported outside of the UK.
| Health marks which apply from 1 January 2021 | Establishment where mark is applied | Great Britain market | Northern Ireland market | EU 27 market | Non-EU market |
|---|---|---|---|---|---|
 |
Great Britain - FSS approved establishments |
Yes | Yes | Yes | Yes* |
 |
Great Britain - FSS approved establishments |
Yes | Yes | Yes | Yes* |
 |
Great Britain - FSS approved establishments |
Yes | No | No | Yes* |
Size and dimension of health marks from 1 January 2021
The health mark must:
- be a legible and indelible oval mark at least 6.5 cm wide by 4.5 cm high
- contain either the ‘GB’ or ‘UK’ abbreviation or the full country name ‘UNITED KINGDOM’ in capitals followed by the approval number of the establishment
- have all letters at least 0.8 cm high
- have all numbers at least 1 cm high
The ink used for the health mark must be authorised in accordance with food law which governs the use of colouring substances in food.
The dimensions and characters of the health mark may be reduced for health marking of lamb, kids, and piglets.
Application of health marks
Food Standards Scotland (FSS) enforcement officials are responsible for the application of the health mark to carcasses in approved slaughterhouses and game handling establishments in Scotland. FSS officials will apply the GB health mark unless advised by a food business that an alternative mark is required for their intended market.
Adjustment period for changes to health marks for POAO placed on the Great Britain market
The 36-month adjustment period in which it has been possible to continue using the ‘UK/EC’ health mark for products being placed on the GB market, will expire on 31 December 2023. After this date, only health marks contained in Table 2 above may be applied.
Products placed on the market after EU Exit
POAO moved into the EU and Northern Ireland markets from Scotland need to be labelled in line with EU requirements. The EU has advised that the official two-digit ISO Code i.e. “GB”, or country name written out in full must be used for products moved into the EU and Northern Ireland markets from GB.
Food businesses should discuss and confirm the use of the appropriate identification mark with their enforcement/Export Health Certificate (EHC) certifying officer, where a specific mark is required for export in accordance with third country requirements.
To note, there is no change in requirements relating to businesses receiving carcasses and cuts of red meat bearing an EC health mark for further processing and export. Approved establishments receiving such product should continue to process and apply their own identification mark in accordance with the regulations.
Find local authority contact details.
See lists of professionals available to sign Export Health Certificates (EHCs).
For further information on 3rd country export certification requirements more generally please contact the Animal and Plant Health Agency.
Summary and actions
Businesses must continue to make sure that only safe food is placed on the market. They should also be mindful that their product needs to comply with any additional marking requirements set by the importing country.
The EHC certifying officer, in order to complete the EHC, will need to attest that the correct identification mark is applied (amongst other things).
Businesses may overwrap products as long as they have been approved and it is their mark that is being applied. However, they should be aware that this may carry a risk of rejection on border inspection should any overwrapping come loose or be damaged in transit.
If you have any queries about the content of this guidance please contact your enforcement officer/OV in the first instance.
Health and ID marking for exports to the non-EU market may depend on the requirements set by the importing nation. Whilst it is anticipated that any of the above marks will be considered acceptable by non-EU third countries, please check with your importer that the relevant requirements for marking and export more generally are being met.
Post EU Exit
Changes to the regulatory and business environment.
EU Exit - Registration and Inspection of Fishing Vessels
Letter for Fishing Vessel Owners in Scotland