In Summary
- Below is a list of commonly asked questions relating to environmental sampling of smoked fish plants.
Questions and answers relating to environmental sample collections in plants manufacturing cold smoked fish
Q. How often should plant environmental samples be taken?
A. The frequency of sample collections is decided by the food business operator (FBO). Many FBOs review their sampling frequencies on the basis of their historical results. Once a history of satisfactory results is established, it can be used as justification for reducing the numbers of samples collected and the frequency of testing. For those businesses that are starting to test, general advice is that they may want to consider collecting 10 samples every fortnight as a starting point, although there are no legally described initial sample collection frequencies. FBOs should be aware it is considered a good practice to vary the day of the week that environmental samples are collected. Article 5 of EU regulation 2073/2005 states that “Food business operators manufacturing ready-to-eat foods, which may pose a Listeria monocytogenes risk for public health, shall sample the processing areas and equipment for Listeria monocytogenes as part of their sampling scheme.”
Q. What kinds of bacteria are counted from environmental swabs?
A. The FBO should decide the types of bacterial tests to carry out. Plants that want to check cleaning effectiveness routinely count glossary item total aerobic mesophiles. Cleaning effectiveness samples are normally collected after the completion of cleaning and just before the commencement of a day’s processing. Listeria monocytogenes testing can also be undertaken to assess cleaning effectiveness. There is no legal requirement to undertake testing for TAM. However, EC 2073/2005 article 5 confers a legal obligation on FBOs to test processing environments used to manufacture ready-to-eat foods for L. monocytogenes. In practice, most FBOs test for general Listeria species (because it is cost effective to test for Listeria generally). If there is a Listeria isolation, the FBO can then have it typed to species level to determine if it is L. monocytogenes.
Those plants that isolate L. monocytogenes from cleaning effectiveness samples, will want to investigate the sources of the contamination. As part of those investigations, environmental samples can be collected during processing to pinpoint any L. monocytogenes niches [glossary entry niche] that are ineffectively cleaned. Isolates from samples collected during processing can by typed to determine if they are plant resident strains.
Q. Where should I take samples from?
A. The location of the sample collections is decided by the FBO. As a rule of thumb, many businesses decide that approximately two thirds of the samples should be taken from food contact surfaces on processing equipment such as converyor belts, knives, skinning and slicing equipment. One third of samples should be taken from elsewhere in the plant environment such as drains, door handles and equipment switches and controls. The surfaces to be tested should be ones that are routinely cleaned and disinfected (see below).
Q. When should I take my samples?
When samples are collected, depends on what the FBO wants to determine. For cleaning effectiveness assessments, most FBOs clean down their plant at the end of a day’s processing. Surface sampling however is commonly delayed until just before the commencement of the next day’s processing. Commonly that can be 5-12 hours after cleaning has finished. The reason is that the FBO wants to determine if the surfaces are fit for processing. It is common to create aerosols when cleaning and if there are bacteria in the aerosols, they can settle out of the air onto surfaces over time, contaminating them.
If a FBO is routinely isolating L. monocytogenes from surfaces or products, they may wish to collect samples from food contact surfaces during processing. The strategy is designed to identify if there are places in equipment (inside conveyor belt rollers or in moving joints and hinges) that do not get effectively sanitised and release L. monocytogenes from these sheltered areas onto products only during processing when the equipment is operating.
Q. Can you give me some examples of where I should take samples from?
A. A list of locations likely to harbour L. monocytogenes and how other FBOs have successfully decontaminated equipment and environments is provided on this page (hyperlink to the “previously successful decontaminations of equipment and likely niches” document)
Q. What equipment do I need to be able to take an environmental swab sample?
A. It is likely that your testing laboratory will supply the sampling consumables and there might be minor differences in the supplied equipment, with minor cascading changes in sample collection methodologies. There are a variety of ways to collect environmental samples. Two common methods are using drumstick swabs and sponge swabs. Drumstick swabs are commonly used to collect samples from impervious food contact surfaces, such as stainless steel. Sponge swabs tend to be used to collect samples from porous or cracked hard surfaces such as brick and concrete.
Typical equipment is shown in the picture below.

Q. How do I take a sample using drumstick swabs?
1. Put on the gloves.
2. Take an alcohol-soaked wipe and wash your hands with the wipe. Make sure you get in-between your fingers and also the tips of your fingers as shown in the picture below.

3. Take a fresh alcohol wipe and clean off the template and the blades of a pair of scissors.

4. Put the scissors and template down on top of a fresh alcohol wipe to make sure that they don't get re-contaminated.
5. Open two swabs. Keep hold of one (and keep it dry). Dip the other in the neutraliser for at least 10 seconds. (NB: a neutraliser is required to neutralise any sanitiser residues and ensure that any collected bacteria are not killed by these residues on the way to the lab. Guidelines by the EURL lists neutralisers for various biocides.)
6. Squeeze the end of the swab against the inside of the tube of neutraliser to remove excess fluid.
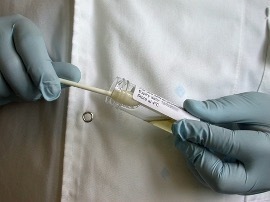
7. Throw away the opened neutraliser.
8. Hold the sterilised template onto the surface to be swabbed
9. Begin with the wet swab. Use a firm pressure and roll the shaft of the swab between the fingers and thumb to rotate the swab whilst rubbing.
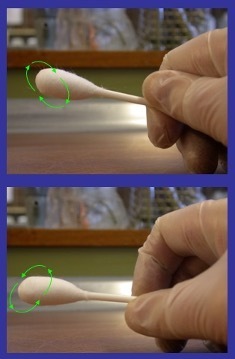
10. Rub the wet swab across the inside of the template. Rub horizontally, then vertically then diagonally as shown below.
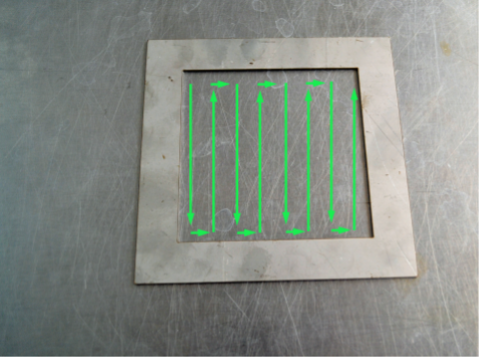
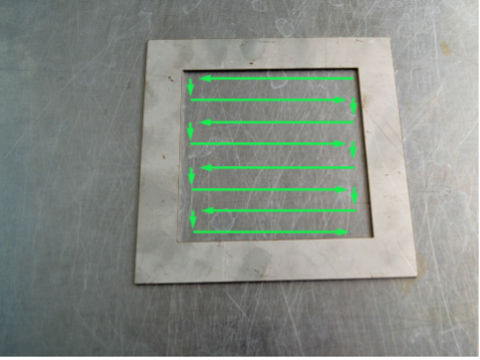
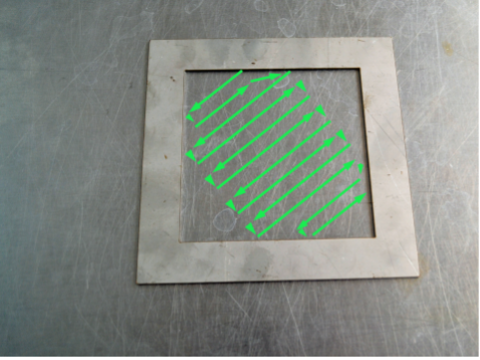
11. Keep the wet swab in your hand. Rub the dry swab across the inside of the template. Rub horizontally, then vertically then diagonally as shown above.
12. It is common for people to use a template when they first being collecting environmental samples. However, within a short period of time, most people acquire an ability to predict where the swab would hit the edge of the template, without the template being present. Since imperfectly sanitised templates can increase the test count, many experienced samplers dispense with using a template.
13. Cut the swabs off into a tube of peptone salt solution (peptone salt solution is also called MRD)

14. Screw the top onto the sample tube, and then label the sample with the date, a description of what was sampled and the size of template used.

15. Immediately put the sample into an insulated cool box containing frozen freezer blocks wrapped in bubble wrap, or crushed ice. Keep the samples chilled (but do not allow them to freeze). Keeping samples cold will prevent bacteria from multiplying and make sure your counts accurately reflect the bacteria present on the surface.
16. When all samples have been collected, package up the samples for sending to the lab. Make sure samples will stay at 0oC to 4oC. Samples should arrive at the lab a maximum of 24 hours after being taken. Transport in a cool box, but do not let the samples freeze or come into direct contact with any frozen blocks.
Q. How do I take a sample using sponge swabs?
A. If the use of gloves is required, put on the gloves and take an alcohol-soaked wipe and wash your hands with the wipe. Make sure you get in-between your fingers and also the tips of your fingers as shown in the picture below.

2. Pour two vials of neutraliser (roughly 40mls) into the bag containing the sponge swab and let the sponge absorb the liquid for at least one minute. (NB: a neutraliser is required to neutralise any sanitiser residues and ensure that any collected bacteria are not killed by these residues on the way to the lab). The sponge should be saturated and drip neutraliser when it is ready for use.

3. Grasp the bottom of the bag containing the sponge and fold the bag back over your hand (Figure A below) so that the sponge is exposed to the air and the bag protects the sponge from contamination from the sampler’s hand (Figure B below).


4. Place the sponge on the porous area to be sampled and press onto the sponge so that the neutraliser is expelled.

5. Relax the pressure on the sponge so that the neutraliser is re-absorbed back into the sponge. Continue expelling and reabsorbing the neutraliser into the surface/into the cracks at least 10 times. If the surface is not too abrasive (e. g. vinyl or wood), the sponge can be squeezed out into the bag and then rubbed across the sampled area in two perpendicular directions to mop up any excess diluent that was not re-absorbed. Rubbing sponges across abrasive surfaces such as concrete however, can cause physical damage and reduce the number of bacteria captured (see below)

Please note, many processors will re-clean and sanitise sampled surfaces to remove chemical residues and potential sources of nutrients for bacteria. Re-cleaning and re-sanitising is considered a good practice.